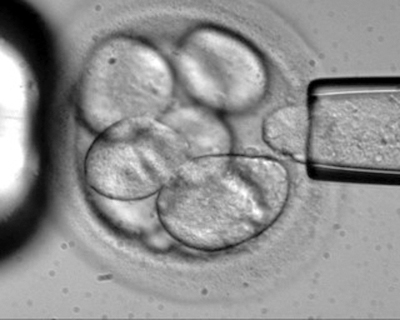
US (n.美國) doctors have begun the first official trial (n.試驗) of using human embryonic (adj.胚胎的) stem (n.幹) cells in patients after getting the green light (n.批准) from regulators (n.規管當局).
The Food and Drug Administration (n.美國食品及藥物管理局) has given a license (n.許可證) to Geron to use the controversial (adj.引起爭議的) cells to treat (v.治療) people with spinal (adj.脊椎的) injuries.
The cells have the potential (n.可能性) to become many of the different cell types found in the body, including nerve (n.神經) cells.
The trials at a hospital in Atlanta (n.亞特蘭大) will check if the treatment is safe.
Geron, a biotech (n.生物科技) company based (adj.位於……的) in "silicon (n.矽) valley" south of San Francisco (n.三藩市), has spent $170m on developing a stem cell treatment for spinal cord (n.脊髓) injury.
The research will use cells coaxed (v.勸誘) to become nerve cells which are injected (v.注射) into the spinal cord.
In animal trials of the treatment, paralysed (adj.癱瘓的) rats regained (v.恢復) some movement. But it is not yet known if it will offer any benefit to people with spinal cord injuries.
It will take some time to get the results. And there are many years of rigorous (adj.嚴謹的) testing ahead (adv.在前面) before it can be known if the therapy (n.治療) is safe and effective.
(BBC, October 11)
美國醫學界得到了規管當局批准後,已在病人身上展開了首個正式的人類胚胎幹細胞試驗。
美國食品及藥物管理局向Geron發出了許可證,批准他們利用這種引起爭議的細胞來治療脊椎損傷。
這種細胞可以發展為身體內諸多不同細胞類型,包括神經細胞。
這次試驗在亞特蘭大一家醫院進行,以研究這種治療方法是否安全。
Geron是一家生物科技公司,位於三藩市南部的“矽谷”。該公司耗用了1億7千萬美元,研製出一種幹細胞治療脊髓損傷的方法。
這項研究將會利用特別方法令幹細胞發展成神經細胞,然後注射到脊髓內。
在這種療法的動物試驗中,癱瘓的老鼠恢復了一些活動能力;但它能否為脊髓損傷患者提供任何益處卻依然未知。
結果要一段時間後才能知曉;而要肯定這種治療是否安全有效,則尚要經過未來很多年的嚴謹測試。
(英國廣播公司,10月11日)